(Editors' Note: This article discusses a micro-cap stock. Please be aware of the risks in these stocks.)
GeneNews Limited (GNWSF.PK) is a molecular diagnostics company focused on developing and commercializing proprietary blood-based diagnostic tests for early disease detection, determining the stage of disease and monitoring its progression, treatment or recurrence.
The Company has developed a powerful approach to identify unique RNA-based signatures from whole blood, the Sentinel Principle?, which has the ability to detect and stage virtually any disease or medical condition from a simple blood sample. The Sentinel Principle? is a platform technology which utilizes functional genomics to enable early diagnosis and personalized health management. Sentinel Principle is proprietary and protected by broad pioneering foundational patents. GeneNews is has applied the Sentinel Principle in major areas with unmet clinical needs such as cancer, arthritis, cardiovascular disease and neurological disorders.
The Company's current efforts are primarily focused on the commercialization of its lead product
ColonSentry? and development of new products in collaboration with research and development partners.
ColonSentry is a blood test to determine an individual's current risk for colorectal cancer ((CRC)). ColonSentry is already available in the US, Canada, Malaysia and China. The Company is actively in discussions with potential partners to establish a global ColonSentry marketing and distribution network that will make this innovative product available to patients in Asia, Europe, and additional regions of the U.S. and Canada.
GeneNews Targets the Large Medical Diagnostics Market
GeneNews is a medical testing service provider specifically focused on molecular/genetic (GM) testing. Recent advances in the genomic and proteomic research, combined with the complete sequencing of the human genome, have made sophisticated new scientific testing tools to diagnose and treat diseases possible, and therefore boosted the lab testing market dramatically.
The clinical medical testing industry consists primarily of three types of providers: hospital-based laboratories, physician office laboratories and independent clinical laboratories. The total medical testing market is a multi-billion dollar business with estimated total revenue of about $62 billion in 2009 and hospital affiliated labs account for about 54% of the market share.
GeneNews is an independent lab testing provider with current focus on cancer diagnostics. The market for cancer testing is growing rapidly due to the following key factors:
- Cancer is the leading cause of death in the US, and one in 4 deaths in the United States is due to cancer. A total of 1,660,290 new cancer cases and 580,350 deaths from cancer are estimated to occur in the United States in 2013 according to American Cancer Society.
- Cancer is primarily a disease of the elderly and now that the baby boomer generation has started to turn sixty, the U.S. is experiencing a significant increase in the number of senior citizens. The American Cancer Society estimates that one in four senior citizens will develop some form of cancer during the rest of their lifetime;
- Every year more and more genes are implicated in development and/or clinical course of cancer. These associations fuel the development of new genetic or molecular tests.
According to Frost & Sullivan, the total cancer testing market is about $10 billion in the US, and grows very rapidly. This market is expected to grow at a healthy compound annual rate of 6.5% despite the present economic uncertainty, impending healthcare reform, and cost/reimbursement issues. GeneNews currently addresses approximately $2 billion of the colon cancer testing market.
The Unique Sentinel Principle? Platform Technology
The key asset of GeneNews is its Sentinel Principle? Platform Technology, a novel approach to identifying biomarkers of body state using blood. This unique blood biomarker approach has been modified and refined with time, but is based on the scientific observation that circulating blood reflects, in a detectable way, what is occurring throughout the body.
The Sentinel Principle? technology is based on the concept that all clinical conditions and body states, including those resulting from disease or in response to treatment, generate characteristic gene expression signatures in the blood as a result of the constant and dynamic physiological interaction of blood with the cells, tissues and organs of the human body. This technology is the basis of GeneNews' first product, ColonSentry? the world's first blood test for colorectal cancer.
One of the strengths of the Sentinel Principle is its flexibility. Applying it to different disease areas enables GeneNews' scientists to generate specific combinations of biomarkers for numerous applications, indeed theoretically virtually any medical condition. This enables GeneNews to focus on the clinical questions and diseases with the greatest unmet need and largest commercial opportunities.
The Sentinel Principle has a broad range of applications including early diagnosis, determining stage of disease, identifying responders/non-responders to a specific therapy, monitoring progression/recurrence of disease, monitoring the effects of treatment, and monitoring treatment compliance.
The Sentinel Platform targets many of the most critical and costly problems currently facing patients, providers and reimbursers of healthcare. GeneNews has identified four major disease groups where advanced diagnostics technology can make a fundamental difference.
Cancer diagnostics is a key area GeneNews is targeting. GeneNews' Sentry products will enable earlier and broader detection of cancer. For example, few adults undergo regular screening for colorectal cancer because traditional screening methods are generally inconvenient, uncomfortable and time-consuming. The development of convenient and accurate blood tests for cancer detection and to facilitate compliance with established screening practices would certainly have a positive impact on public health.
In addition to cancer, GeneNews has also applied its technologies to cardiovascular disease, central nervous system disease, and arthritis.
ColonSentry?: World's First Blood Test To Determine Risk of Colon Cancer
ColonSentry? is GeneNews' first commercial product which is developed based on the Company's Sentinel Principle Technology.
ColonSentry is a sophisticated blood test that can assess a person's current risk of having colorectal cancer. The test is not considered a replacement for colonoscopy, but rather a more convenient step in detecting early warning signs of colon cancer.
ColonSentry does not require a patient to provide a stool sample, nor does it require any dietary restrictions like fasting or refraining from certain foods or medications prior to taking the test. The ColonSentry test is easy to perform and blood can be drawn at the same time as other blood tests with no advanced preparation.
The ColonSentry test measures the expression of seven genes which serve as biomarkers (biological indicators) to detect colorectal cancer risk. The science behind the ColonSentry test is based on the Sentinel Principle?, a breakthrough approach that identifies biomarkers for disease in circulating blood to detect what is occurring throughout a person's body.
The 7-gene biomarker panel was developed from the training set of 232 samples (112 CRC and 120 controls), and validated from the test set of 410 samples (202 CRC/208 controls). The Company analyzed 196 gene expression profiles from the above 642 samples to select candidate CRC biomarkers. Seven genes were selected for the development of CRC biomarker panel. Six of them (ANXA3, CLEC4D, LMNB1, PRRG4, TNFAIP6 and VNN1) were overexpressed (1.31- to 1.67-fold), and 1 (IL2RB) was under-expressed (0.84-fold) in CRC when compared with controls.
(click to enlarge)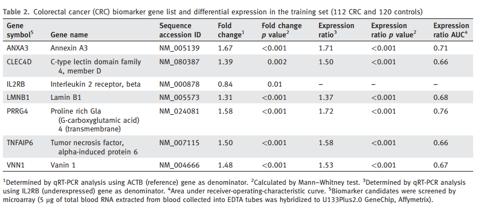
Panel performance characteristics and disease prevalence (0.7%) were then used to develop a scale assessing an individual's current relative risk (CURR) of having CRC based on his/her gene signature. Individual gene profiles were compared against the population results and used to calculate the current relative risk for CRC.
Bayes' theorem was used to calculate an individual's CURR, de?ned as the ratio of the probability of having CRC to the CRC prevalence, based on their blood-sample gene expression pro?le. At CURR=1, a subject has the same CRC risk as the unstrati?ed average-risk population. At CURR=10, the subject has a 10-fold risk increase. Similarly, at CURR=0.1, the subject has a 10-fold risk decrease.
The performance of the predictive model on the training set had the following characteristics: 73% accuracy, 82% sensitivity, 64% speci?city, 68% positive predictive value (PPV) and 79% negative predictive value (NPV). The performance of the predictive model on the test set had the following characteristics: 71% accuracy, 72% sensitivity, 70% speci?city, 70% PPV and 73% NPV.
The differentiated expression of the 7 genes forms the basis of the ColonSentry test. The 7-gene panel can stratify subjects according to their current relative risk across a broad range in an average-risk population. Across the continuous spectrum of risk as defined by the current relative risk scale, it is possible to identify clinically meaningful reference points that can assist patients and physicians in CRC screening decision making.
(click to enlarge)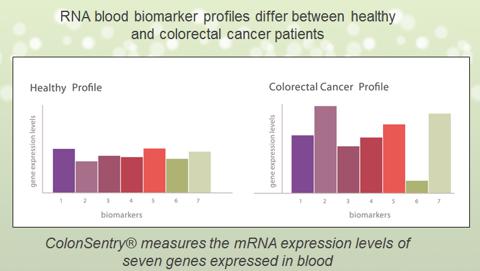
Colorectal cancer is the number two cause of cancer death in men and women. Because this deadly disease shows no symptoms in its most curable stages, early detection can increase a patient's chances of survival.
Huge Market for ColonSentry
The CRC Background
According to National Cancer Institute (NCI), colorectal cancer (CRC) is the third most common malignant neoplasm and the second leading cause of cancer deaths (irrespective of gender) in the United States. It is estimated that there will be 142,820 new cases diagnosed in the United States in 2013 and 50,830 deaths due to this disease. About 5% of Americans are expected to develop the disease within their lifetimes.
Worldwide, CRC also ranks the third most common cancer according to Cancer Research UK. An estimated 1.24 million people worldwide were diagnosed with colorectal cancer in 2008, accounting for 10% of the total. CRC is the fourth most common cause of cancer death worldwide, estimated to be responsible for almost 610,000 deaths in 2008.
The risk of CRC begins to increase after the age of 40 years and rises sharply at ages 50 to 55 years. The risk doubles with each succeeding decade, and continues to rise exponentially. Despite advances in surgical techniques and adjuvant therapy, there has been only a modest improvement in survival for patients who present with advanced neoplasms. Hence, effective primary and secondary preventive approaches must be developed to reduce the morbidity and mortality from CRC.
CRC incidence and mortality rates have been declining for the past 2 decades in the US in both men and women, which is largely attributable to the contribution of screening to prevention and early detection. However, there are still about 56% of people at high risk of CRC who don't get screened. And about 60% of CRCs are detected too late.
CRC Screening
Colorectal cancer (CRC) screening can detect cancer. If CRC screening reveals a problem, diagnosis and treatment can occur promptly. In addition, finding and removing polyps or other areas of abnormal cell growth may be one of the most effective ways to prevent CRC development. Also, CRC is generally more treatable when it is found early, before it has had a chance to spread.
Current recommended CRC screening tests are grouped into 2 categories:
- Tests that primarily detect cancer, which include both the guaiac-based fecal occult blood test (gFOBT) and fecal immunochemical test (FIT, also called immunochemical fecal occult blood test) and testing stool for exfoliated DNA;
- Tests that can detect cancer and advanced lesions, which include the endoscopic examinations and radiological examinations including flexible sigmoidoscopy (FSIG), colonoscopy, double-contrast barium enema (DCBE), and computed tomography colonography (CT colonography or virtual colonoscopy).
But the most often used CRC screenings are FOBT and colonoscopy. Both have shortcomings. For example, FOBT fails to detect most polyps and some cancers; False-positive results (the test suggests an abnormality when none is present) are possible; Dietary restrictions may be needed before the test; Additional procedures, such as colonoscopy, may be needed if FOBT indicates an abnormality. For colonoscopy, it may not detect all small polyps, nonpolypoid lesions, and cancers; Thorough cleansing of the colon is necessary before this test; Some form of sedation is used in most cases; Although uncommon, complications such as bleeding and/or tearing/perforation of the lining of the colon can occur; Colonoscopy also has a 0.5% incidence of colonoscopy-associated morbidity.
ColonSentry Advantages
Colon Cancer is one of the most preventable and treatable forms of cancer when detected early. According to the American Cancer Society:
- The 5-year relative survival rate for people whose colorectal cancer is treated in an early stage is better than 90%
- The 5-year relative survival rate if cancer has spread to distant organs (i.e., the liver or lung) is less than 10%
- Only 40% of colorectal cancers are found in early treatable stages.
Therefore, early diagnosis and treatment is the key to improve CRC survival. Unfortunately, since traditional tests for colorectal cancer including FOBT and colonoscopy are often perceived to be inconvenient and uncomfortable, people avoid or delay being tested until symptoms appear which usually means the cancer is in its late stages.
CRC screening saves lives, but patient compliance with faecal testing and endoscopy remains low. Although colonoscopy is considered a CRC diagnostic ''gold standard,'' as a screening tool the technology has limitations. Many are averse to the procedure, and most healthcare systems have limited capacity. Even in the United States, colonoscopy capacity is insuf?cient to adequately screen the entire average-risk population. Furthermore, the 0.5% incidence of signi?cant colonoscopy-associated morbidity is of concern given low CRC prevalence (0.7%) in the over 50, average-risk population.
(click to enlarge)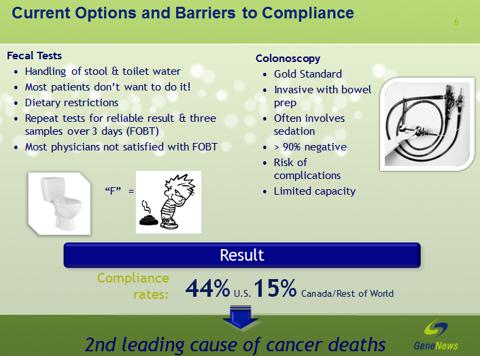
GeneNews' ColonSentry is a blood-based test which can provide clinically actionable CRC risk information. ColonSentry will likely enhance screening compliance and facilitate clinical decision making. The 7-gene ColonSentry test can be incorporated into CRC decision making in several ways.
- A blood-based ColonSentry test would bene?t patients who desire information about their CRC status but refuse screening due to dislike of screening options. In particular, identi?cation of increased current CRC risk may facilitate colonoscopy decision making for these patients, who would otherwise refuse colonoscopy.
- Second, in healthcare systems with limited colonoscopy capacity, blood-based ColonSentry could help prioritize patients at greatest current risk for CRC, similar to the proposed breast cancer strati?cation strategy. Combining ColonSentry testing in advance of colonoscopy can detect 2.1-4.7 times more cancers, when colonoscopy capacity is between 10% and 40%, which is the case in most countries.
- Furthermore, identifying patients with diminished current CRC risk with ColonSentry can help enhance physician and patient decision making. Provision of this type of novel, decreased-risk information can help facilitate subsequent screening decision making that is tailored to a patient's individual circumstances. It can also help ensure that ?nite colonoscopy resources are directed to those with greatest risk.
In sum, the blood-based 7-gene biomarker test ColonSentry provides enriched information about an individual's CURR for having CRC. As a blood test, it addresses one of the greatest challenges currently limiting CRC screening effectiveness: lack of compliance. Additionally, by identifying patients with enhanced CURR and with diminished CURR, ColonSentry can help healthcare providers assess need for increased monitoring or further workup, and help tailor the use of invasive and expensive procedures to those most likely to bene?t.
A health economic analysis on ColonSentry demonstrated that screening with the ColonSentry test lowered the costs associated with CRC by increasing early-stage CRC detection and extending survival. From the private payer perspective, ColonSentry was dominant (produced better patient outcomes at lower costs) over no screening and FOBT. From the healthcare perspective, the ColonSentry test is cost-effective compared with the generally accepted oncology ICER benchmark. Screening is even more cost-effective for all perspectives when patients have the option of either FOBT or the ColonSentry test.
Although launched recently, ColonSentry is already moving patients toward colonoscopy compliance.
The Market Potential
The annual expenditure for colorectal cancer treatment in the US was conservatively estimated at $14 billion in 2010 by the National Institutes of Health (NIH) and that is estimated to increase to $17 billion by 2020. ColonSentry is targeting the huge CRC early screen market with $2 billion market opportunity.
The US opportunity for ColonSentry
(click to enlarge)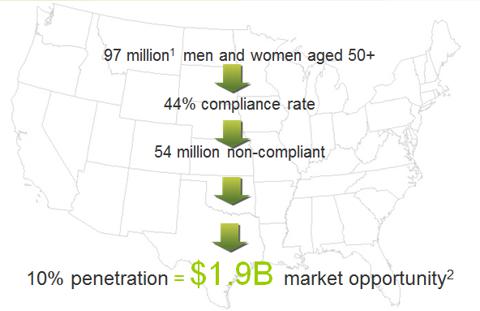
Going Global, the Ultimate Marketing Strategy
GeneNews is a Canadian company, but it is working with its partners in other countries to make ColonSentry available to as many people as possible. ColonSentry? is now available in Canada, USA, Malaysia and China.
Certainly, the US market is the most important one for GeneNews.
On April 10, 2012, the Company announced the commercial launch of its lead product, ColonSentry?, by its first U.S. marketing partner, Enzo Clinical Labs (a division of Enzo Biochem, Inc.), into the New York and New Jersey region.
On July 2, 2013, GeneNews formed a joint venture with two private American companies, Health Diagnostic Laboratory and a sales organization with national capabilities, to establish an accredited clinical laboratory called Innovative Diagnostic Laboratory, LLP (IDL). IDL will initially focus on the development and broader US commercialization of ColonSentry and is expected to be functional and certified under the Clinical Lab Improvement Amendments Act (CLIA) in the third quarter.
GeneNews has granted IDL an exclusive license for certain technology related to the ColonSentry test for immediate development and commercialization throughout the United States, excluding NJ and NY. Each party to the JV will receive a share of the revenues and profits generated by IDL.
The launch of ColonSentry in the US is a significant milestone for the commercialization of GeneNews' proprietary Sentinel Principle? platform technology. Currently, the Company's activities have been primarily focused on U.S. ColonSentry? commercialization initiatives that include the development of a reimbursement strategy, the planning and initiation of additional clinical studies, and evaluation of potential technology platforms to reduce costs for next generation ColonSentry? and pipeline products.
We are encouraged by the positive response to the recent launch of ColonSentry? into New York and New Jersey by the Company's first U.S. partner. The establishment of IDL provides the Company with a US commercialization platform for the introduction of ColonSentry into the broader US market. As a result, revenue growth will accelerate in the coming quarters.
China is another important market for ColonSentry.
In September 2012, GeneNews entered into a strategic alliance with Shanghai Biochip Co. Ltd. (SBC). Shanghai Biochip is a national engineering center for advanced microarray and gene expression profiling technologies in China that specializes in genetics, protein and other micro-organism detection with a dedicated clinical diagnostics institute that provides molecular examination services for early diagnosis and personalized treatment of certain diseases.
GeneNews and SBC will establish the first Sentinel Centre for Personalized Medicine, based in Shanghai, to co-develop and commercialize additional products based on GeneNews' Sentinel Principle? Technology. For 2013, the Company has targeted the initiation of a program to develop a test to facilitate the early detection of lung cancer and expects to define the study protocol and commence sample accrual from multiple centers in China. Under the terms of the agreement, SBC also obtains non-exclusive rights to market and sell ColonSentry? test in China.
With an annual GDP growth rate over 7.5% in the past 30 years, China has been one of the major pharmaceutical markets in the world. One of the focuses of Chinese government is to enhance and improve health standards for its 1.3 billion people population, particularly with respect to the prevention, early detection and personalized treatment of diseases such as cancer, diabetes and mental disorders.
The cooperation with SBC represents a significant milestone for the commercialization of ColonSentry, opens the door to the large Chinese market. Going forward, China will be a significant revenue source for GeneNews' ColonSentry and other pipeline products based on the Sentinel technology.
ColonSentry is also available in Canada and Malaysia.
GeneNews is Undervalued
GeneNews is a commercial stage molecular diagnostics company with a current focus on cancer detection. GeneNews has developed a unique Sentinel Principle platform technology based on functional genomics. Based on this technology, the Company has recently launched its lead product ColonSentry for early screening of colorectal cancers (CRC) and SentinelGx services.
We think revenue will accelerate in the coming years thanks to its focused marketing strategy and continued new products/services offering. Currently, ColonSentry is the main driver for revenue growth. We see total revenue growing at an impressive 156% compound annual growth rate (CAGR) from fiscal 2013 to 2018 according to our financial model. We model that the Company will become profitable in fiscal 2017 with earnings per share (EPS) of $0.05 based on total revenue of $19.00 million. We forecast EPS will grow to $0.16 per share based on revenue of $28.5 million in fiscal 2018. This is impressive considering the relatively short history of the operations and the small size of the Company.
Based on GeneNews' strong fundamentals, we think the Company is undervalued. Currently, GeneNews shares are trading at about $0.90 per share which values the Company at $30 million in terms of market cap based on 33.7 million shares outstanding. This is a deep discount compared to its peers in our view. Based on our financial model, revenue will grow at amazing 156% CAGR from 2013 to 2018. GeneNews will become profitable in 2017. We think GeneNews shares should trade at 45 x P/E multiple which is similar to the biotech industry average P/E ratio. This P/E is justified because GeneNews will experience a high growth in the next five years. If we use this P/E multiple, coupled with our estimated EPS of $0.16 in 2018, discounted at 20% for four years, we come up with a price target of $3.50 per share.
One wild card for GeneNews valuation is that the Company could be an acquisition target for big players. The clinical lab testing industry is quite fragmented currently, and merger & acquisition activity is looming. We have noticed that big players LabCorp and Quest Diagnostics are increasingly acquiring smaller players in this field. Qiagen NV, a research service company based in Netherland, entered into molecular diagnostics market in 2007 by acquiring Digene Corp. Since then, Qiagen has been quite aggressive in acquisition of other small genetic/molecular testing companies.
With the increased activity in M&A in the industry, GeneNews could be an easy target for acquisition. If acquired by big players, share price of GeneNews may soar.
We are optimistic about the Company's prospect. With a rapidly growing cancer testing market worldwide, combined with its unique technology and broad range of product offering, the Company is well positioned to boost its top line and bottom line in the coming years. We think at this time, downside risk for GeneNews is relatively low while upside potential is high.
Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Presidents Day 2013 jack white wiz khalifa 2013 Grammys kelly clarkson Lumineers The Lumineers
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.